Virtue® SAB is investigational and not commercially approved.
Virtue SAB is designed to enable protected drug delivery and extended focal release of therapeutic levels of sirolimus over the critical healing period, revolutionizing drug delivery while leaving nothing behind.

Precise dose is loaded and protected in dose unit (DU)
No drug loss in transit
Balloon positioned using standard PCI technique no 'rush to target' as dose is protected inside DU
No time constraints on positioning
Virtue® SAB inflated to simultaneously dilate the vessel and deliver intended dose through the micropores.
No drug coating particulates
Design to support extended focal release of therapeutic levels of sirolimus through the critical healing period.
Virtue® SAB is investigational and not commercially approved.
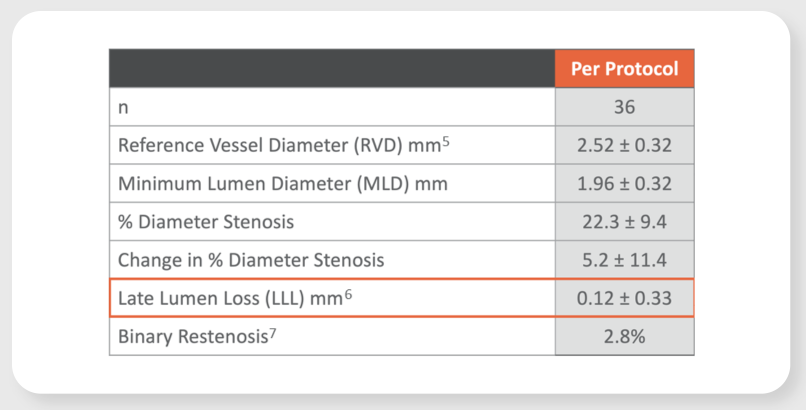
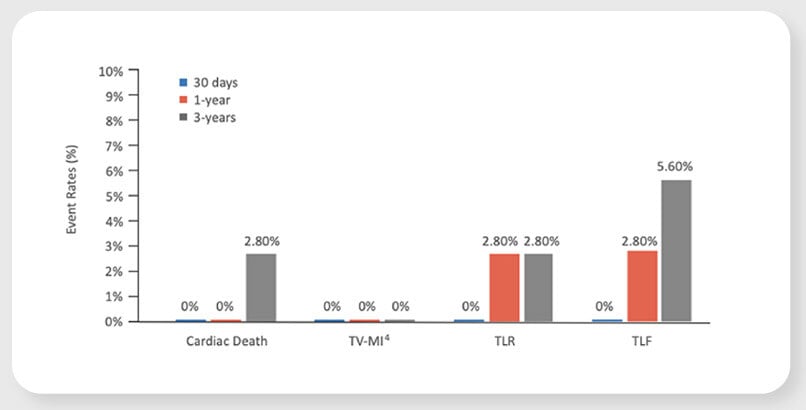
Virtue SAB is supported by encouraging published preclinical data and clinical data out to 3 years.
Virtue SAB preliminarily promising safety and efficacy results in patients with coronary in-stent restenosis (ISR) in the prospective, multi-center SABRE Trial
LLL at 6-month angiographic assessment
TLF4 at 1 year
new TLR between 1 to 3 years
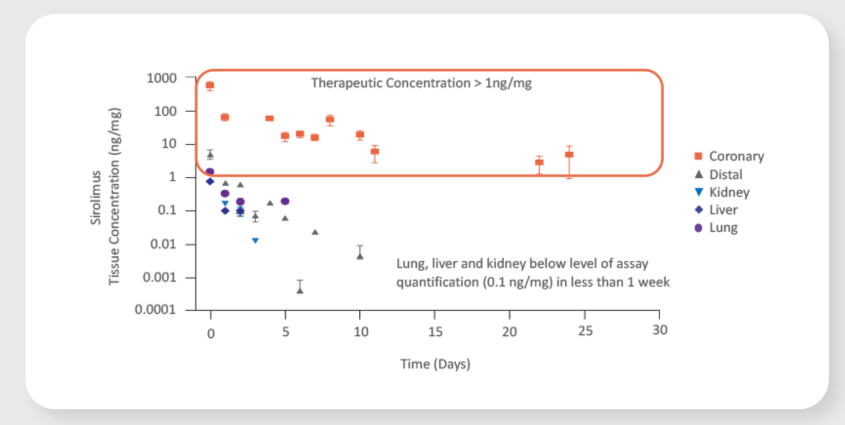
Virtue SAB achieved focal therapeutic sirolimus tissue concentrations through the critical healing period of approximately 30 days.