Our Innovations
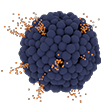
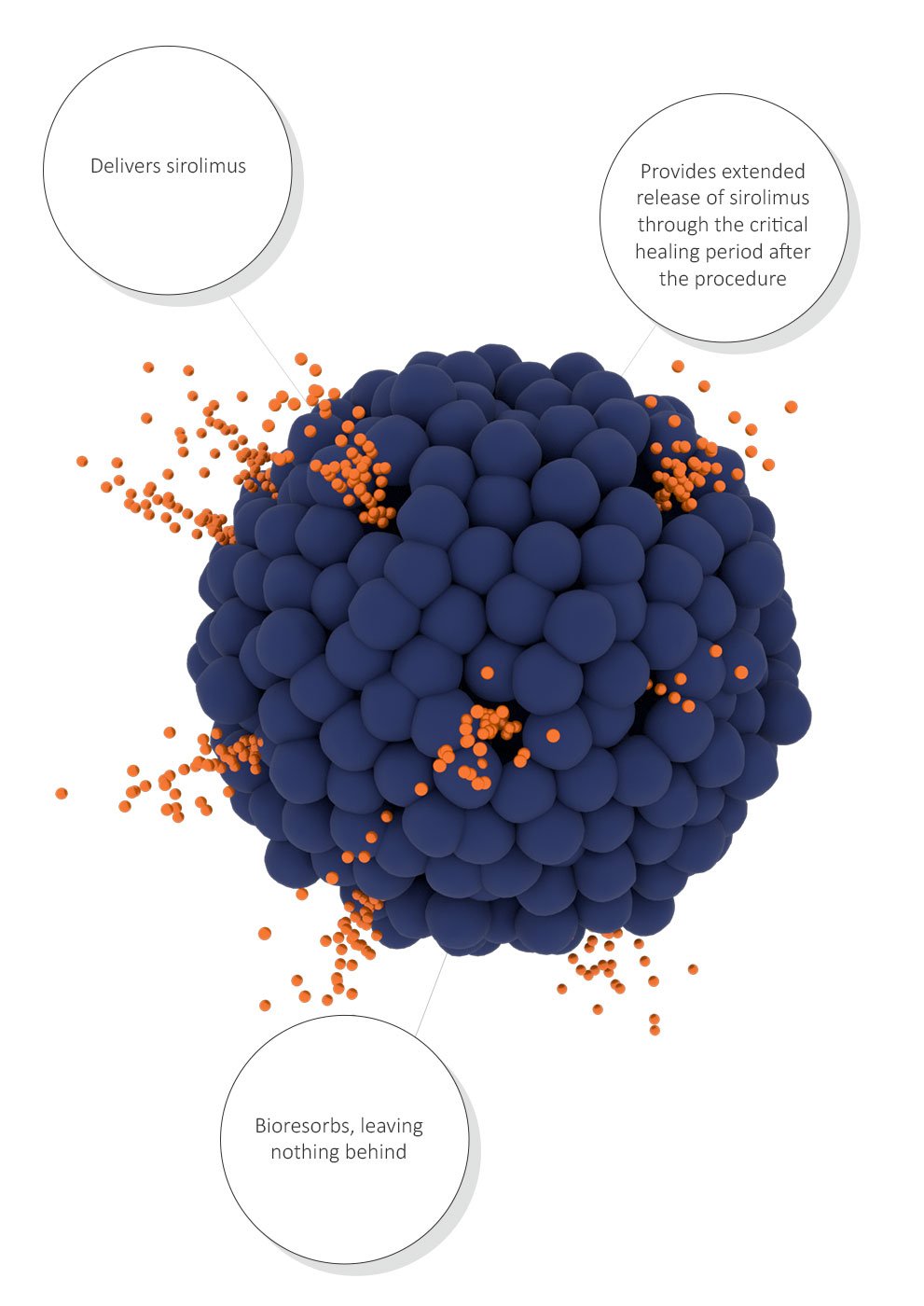
Virtue SAB is designed to enable protected drug delivery and extended focal release of therapeutic levels of sirolimus over the critical healing period, revolutionizing drug delivery while leaving nothing behind.

Designed to...
Dilate artery and restore blood flow without leaving a permanent implant behind.
Enable protected drug delivery to consistently deliver the intended dose and to reduce potential downstream ischemia from large drug coating particulates.
Designed to...
Deliver sirolimus, a proven anti-restenotic, cytostatic drug with a broad safety window.
Provide extended focal release of therapeutic levels of sirolimus during the critical healing period of approximately 30 days.
Designed to enable angioplasty with protected drug delivery to overcome challenges of drug-coated balloons.
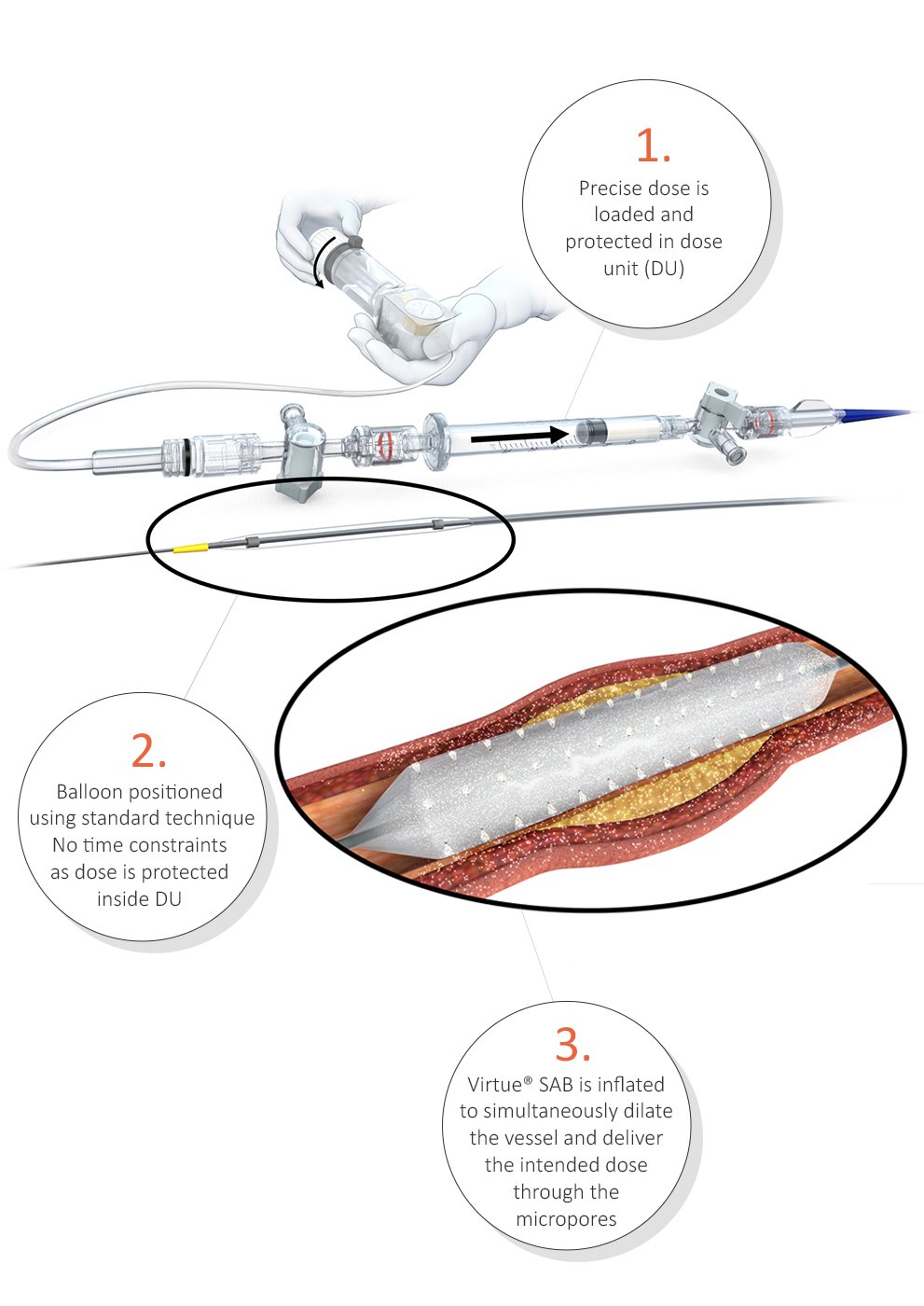
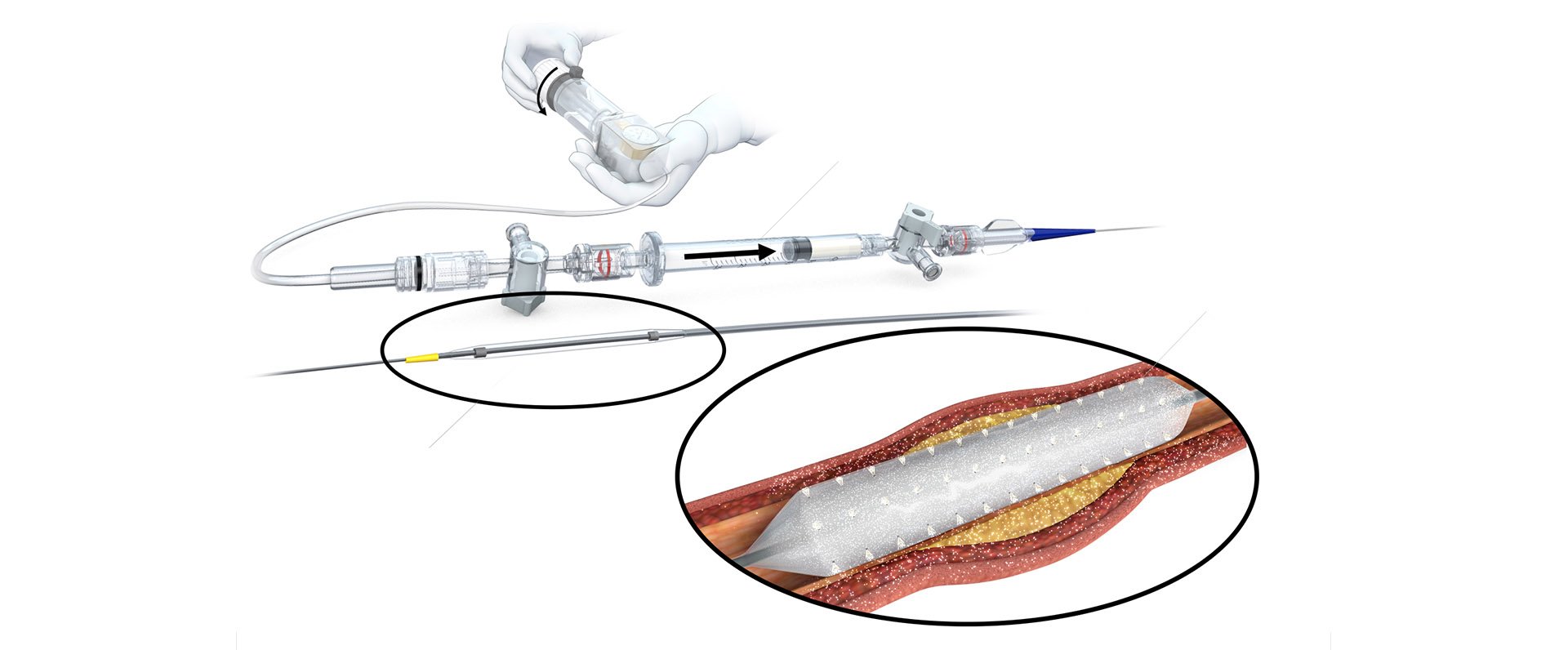



Protected Delivery Design (Non-Drug Coated)
Designed to support extended focal release of therapeutic levels of sirolimus through the critical healing period.



Virtue SAB is supported by encouraging published preclinical data and clinical data out to 3 years.
Virtue SAB preliminarily promising safety and efficacy results in patients with coronary in-stent restenosis (ISR) in the prospective, multi-center SABRE Trial.1,2,3
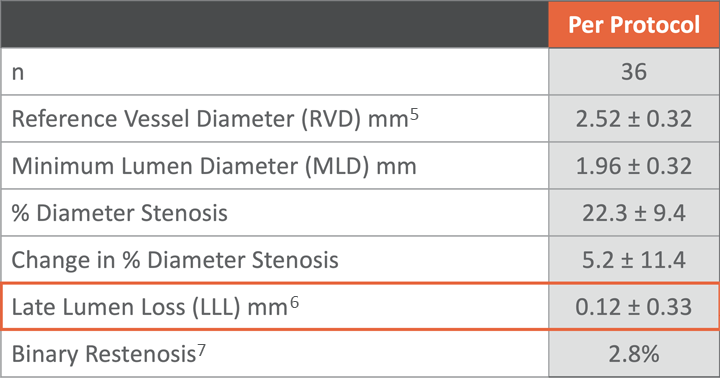
LLL4 at 6-month angiographic assessment
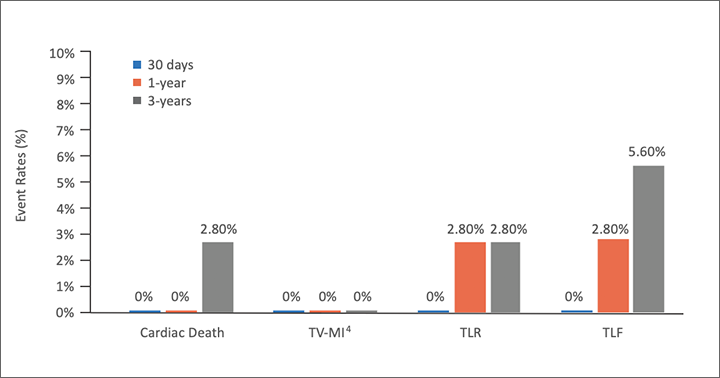
TLF4 at 1 year
new TLR4 between 1 to 3 years
Preliminarily Demonstrated Efficacy
with Low 0.12mm Late Loss
Preliminarily Demonstrated Safety
with Low Event Rates Out to 3 Years
3-Year Clinical Results presented at Transcatheter Cardiovascular Therapeutics (TCT) 2018
Virtue SAB achieved focal therapeutic sirolimus tissue concentrations through the critical healing period of approximately 30 days.8
Enabled Therapeutic Arterial Tissue Concentrations
with Low Systemic Concentrations
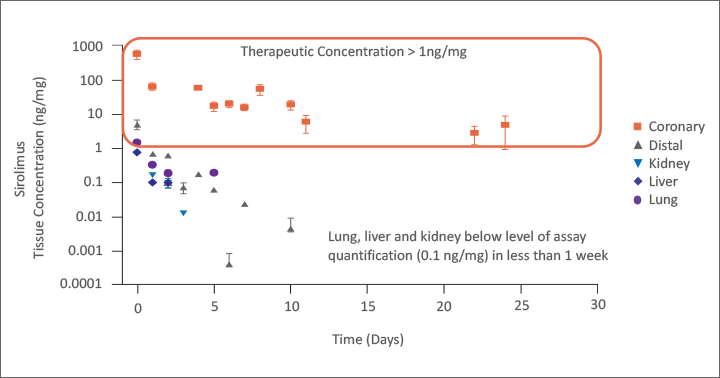
126 pigs, N= 753 arterial segments
1Verheye et al. JACC Cardiovasc Interv 2017 Oct 23;10(20):2029-2037. DOI: 10.1016/j.jcin.2017.06.021.
2Granada 3-Year Clinical Results TCT 2018. 3-Year SABRE Trial Clinical Report on file.
3Revised per protocol analysis set meets the criteria of the proposed In-Stent Restenosis IDE study population
4Target lesion failure (TLF), late lumen loss (LLL), target lesion revascularization (TLR) and Target Vessel Myocardial Infarction (TV-MI)
5RVD reported using Internormal values
6Trial primary performance endpoint
7Trial secondary performance endpoint (binary restenosis = >50% lumen diameter stenosis)
8Granada J, et al. EuroIntervention 2016;12:740-747
Strategic partnership with Terumo Corporation for the development and commercialization of Virtue SAB.


In June 2019, Orchestra BioMed entered into a collaborative agreement with Terumo Corporation, one of the largest medical device companies in the world with corporate headquarters in Tokyo, Japan, pursuant to which Terumo secured global commercialization rights for Virtue SAB in coronary and peripheral vascular indications.
"We are excited to partner with Orchestra BioMed and secure global rights to Virtue SAB, which we intend to make a flagship therapeutic product…The unique design of Virtue SAB demonstrates Orchestra BioMed’s deep knowledge of the needs of interventional cardiologists and its capability to deliver innovative solutions that have the potential to improve patient outcomes."
James Rushworth,
CEO, Terumo Medical Corporation (North America)
"This strategic partnership is a major milestone for Orchestra BioMed. It validates our differentiated strategy to focus on the development of high-impact therapies while leveraging alliances with established market leaders, like Terumo, to drive global commercialization of our products."
David Hochman,
Chairman, CEO and Founder, Orchestra BioMed
About the Agreement
Virtue® SAB is investigational and not commercially approved.
© 2025 Orchestra BioMed Inc. Virtue®, BackBeat CNT™ FreeHold Duo®, FreeHold Trio® and Orchestra BioMed™ are trademarks of Orchestra BioMed.
All other trademarks are trademarks of their respective owners.
SM-0020 Rev 01