Who We Are
Our expertise and passion is developing product innovations to enhance the lives of patients worldwide.

Focused on
improving outcomes
Driven by knowledge
and expertise

Passionate
about innovation

Determined and
perseverant
Our highly accomplished, multidisciplinary team has extensive experience in all phases of therapeutic device development.
Mr. Hochman has served as Chairman and Chief Executive Officer of Orchestra BioMed since May 2018. He previously served as Managing Partner of Orchestra Medical Ventures, an investment firm focused on creating high-impact medical device therapies.
Mr. Hochman has also served as President of Accelerated Technologies, Inc., a medical device accelerator company now owned by Orchestra BioMed. Mr. Hochman has over 25 years of healthcare entrepreneurial, venture capital and investment banking experience. From December 2016 to September 2023, Mr. Hochman served as a member of the board of directors of Motus GI (Nasdaq: MOTS), a Nasdaq listed medical technology company in which Orchestra has a strategic investment and as its Chairman of the Board from December 2016 to April 2023. Mr. Hochman was a co-founder and until September 2020 served as a board member of Corbus Pharmaceuticals Holdings, Inc. (Nasdaq: CRBP), a clinical-stage company focused on the development and commercialization of novel medicines designed to target the endocannabinoid system. Prior to joining Orchestra Medical Ventures, Mr. Hochman was Chief Executive Officer of Spencer Trask Edison Partners, LLC, an investment partnership focused on early-stage healthcare companies. He was also Managing Director of Spencer Trask Ventures, Inc. during which time he led financing transactions for over twenty early-stage companies raising over $420 million. From 1999 to 2006, Mr. Hochman was a board advisor of Health Dialog Services Corporation, a leader in collaborative healthcare management that was acquired in 2008 by the British United Provident Association for $750 million. From 2005 to 2007, he was a co-founder and board member of PROLOR Biotech, Inc., a biopharmaceutical company developing longer lasting versions of approved therapeutic proteins, which was purchased by Opko Health (NYSE: OPK) in 2013 for over $600 million. Mr. Hochman earned a B.A. with honors from the University of Michigan.
Mr. Sherman has served as President, Chief Operating Officer and a member of the Board of Orchestra BioMed since May 2018. From 2009 until December 2019, Mr. Sherman served as Managing Partner of Orchestra Medical Ventures, an investment firm focused on creating high-impact medical device therapies. He co-founded Orchestra BioMed and its wholly owned subsidiaries Caliber, BackBeat and Freehold.
Mr. Sherman also served as Chief Technical Officer of Accelerated Technologies, Inc. (ATI), a medical device accelerator company now owned by Orchestra BioMed. Mr. Sherman has over 25 years of management and entrepreneurial experience in the medical technology industry spanning interventional cardiology, cardiac electrophysiology, sudden cardiac death, stroke, surgery, GI, and neurovascular therapies. Mr. Sherman served as Chief Executive Officer and a Director of Caliber since 2009. He was a co-founder and served on the Board of BackBeat since 2010. He was a co-founder and served as Chief Executive Officer and a director of FreeHold since 2011. From 2009 until 2016, he served on the board of directors of Vivasure. He served as a director of Motus GI (Nasdaq: MOTS), a Nasdaq-listed medical technology company that Orchestra also has a strategic holding in, from December 2016 to September 2023. From February 2002 until March 2008, Mr. Sherman held various positions in executive management for Cordis Neurovascular (CNV), a Johnson & Johnson company, including Executive Director R&D and Director of Strategic Marketing for stroke products. He had worldwide responsibility for all neurovascular R&D and global strategic marketing responsibilities for the stroke franchise, including budgets, portfolio of products and strategic planning. Mr. Sherman made significant contributions to the design and commercialization of a series of key products including the Enterprise Vascular Reconstruction Device and the Orbit Embolic Coil. From January 1997 until February 2002, Mr. Sherman played an integral role in the formation and development of Revivant Corp (acquired by Zoll) while working with Thomas J. Fogarty, M.D. at Fogarty Engineering. He was Revivant’s first employee and managed the design, development, and testing of the AutoPulse device from concept through market introduction. In addition to the R&D related activities, Mr. Sherman supported the clinical research, marketing, and intellectual property efforts. The AutoPulse is a lightweight, portable tool replacing the need for manual chest compressions during cardio-pulmonary resuscitation (CPR) for patients suffering from sudden cardiac arrest. From January 1995 until January 1997, Mr. Sherman held positions in research and development for Cardiac Pathways Corp., prior to its acquisition by Boston Scientific. Prior to Cardiac Pathways, he worked at Baxter Healthcare. In each of these companies, he participated in the creation, development and launch of products. Mr. Sherman has authored more than 85 U.S. patents with an additional 90+ published applications. He earned a BS degree in Bioengineering from the University of California, San Diego.
Mr. Taylor joined Orchestra BioMed as its Chief Financial Officer (CFO) June 2023. He brings over 20 years of CFO experience with emerging growth companies, the last 15 of which were in the medical device sector.
Prior to joining Orchestra BioMed, Mr. Taylor served as CFO of Motus GI Holdings, Inc. (NASDAQ: MOTS), where he helped lead the company’s 2018 IPO. Prior to Motus GI, Mr. Taylor spent 10 years as CFO and President of Avertix Medical, Inc. (f/k/a Angel Medical Systems, Inc.), where he is still a member of the Board of Directors. Prior to Avertix, he was a Practice Leader for AC Lordi Consulting (now part of BDO USA, LLP) and CFO and Corporate Director of Operations for Safe3w, Inc. (acquired by iPass). Mr. Taylor earned a high-honors MBA with a concentration in Accounting and Finance from Northeastern University and a B.A. in Political Science and Economics from McGill University.
Dr. Mika has served as General Manager and Chief Technology Officer of Orchestra BioMed’s Bioelectronic Therapies development group since May 2018. Since 2011, Dr. Mika served as the CEO of BackBeat Medical, LLC. He was one of the founders of BackBeat and the leader in the development of BackBeat Cardiac Neuromodulation Therapy from concept and design of the device through preclinical and clinical work demonstrating the effect of the therapy in lowering blood pressure.
Dr. Mika has over 25 years of experience in the medical device industry, bringing technologies from concept to commercialization. He managed the development of active implantable devices for the treatment of major diseases, including heart failure, obesity and diabetes. Dr. Mika started his career in 1992 as a leading researcher at Carmel Biotechnologies in Israel (later called Carmel Biosensors), developing an implantable biosensor for continued measurement of glucose. Medtronic Public Limited Company later acquired some of the company’s assets. In 1996, Dr. Mika became the Chief Scientist and one of the founders of Impulse Dynamics, a medical device company developing an implantable cardiac stimulator for the treatment of heart failure. In 1998, Dr. Mika became Impulse Dynamics’ Vice President of Research and Development, responsible for the development of the company’s implantable system and preclinical and clinical evaluation of the device. Dr. Mika was part of the team that secured an option agreement, receiving $250M in cash from Johnson & Johnson and Guidant Corporation for the option to acquire Impulse’s technology. In 2001, Dr. Mika was appointed the General Manager of Impulse Dynamics and managed the initial clinical studies of the company and the CE approval of its device and CCM therapy in Europe. In 2005, Dr. Mika was appointed as the Chief Operating Officer and acting Chief Executive Officer of Impulse Dynamics, leading the company’s large-scale randomized studies in Europe and its IDE study in the United States, obtaining high value DRG reimbursement to the system in Europe, reimbursement codes for the system in the US and initiating the commercialization of the system in Europe. In 2003, Dr. Mika led the development of a bioelectrical therapy for the treatment of obesity and diabetes based on the concept of impulse dynamics therapy. This led to the formation of MetaCure, a developer of implantable devices for the treatment of obesity and diabetes. Dr. Mika served as MetaCure’s Chief Operating Officer in 2005 and was the Chief Scientist of MetaCure until 2007. Dr. Mika received his BSc in Electrical Engineering and his PhD in Biophysics from the Technion – Israel Institute of Technology.
Dr. Stoll has served as our Chief Clinical Officer since April 2022, Dr. Stoll is a medical executive with 19 years of business experience in bringing medical innovations to market. He has extensive knowledge in design and execution of clinical strategies and, over the years, has forged strong relationships with global physician opinion leaders.
Previously, Dr. Stoll served as Vice President of International Clinical Affairs & Cardiovascular Research at MCRA, where he led an international team to build MCRA’s cardiovascular CRO as well as integrated regulatory capabilities in Europe. Prior, he was the CMO at Biosensors International where he led a team of 17 people to develop and execute clinical strategies. Dr. Stoll has also served as Chief Medical Officer (CMO) Cardiology for GE Healthcare and was WW Vice President of Clinical Research at Cordis, Johnson & Johnson Company. Dr. Stoll has been extensively published, with over 70 publications in international peer-reviewed medical journals. Dr. Stoll received his medical degree and board certifications in Nuclear Medicine, Internal Medicine, and Cardiology at Saarland University, Germany, completed his Research Fellowship in Cardiology at Indiana University, and earned a Professor of Internal Medicine title from Saarland University in 2005.
Mr. Little joined Orchestra BioMed as its Executive Vice President of Corporate Development and Strategy in June 2023, bringing an extraordinary depth of knowledge, expertise and relationships in the cardiovascular device industry.
Prior to joining Orchestra BioMed, Mr. Little served as Chief Operating Officer at Neovasc, where he drove its successful acquisition by Shockwave Medical in April 2023. Prior to joining Neovasc in 2019, he spent 25 years in the medical device industry, including as a member of the leadership team at Abbott Vascular, where he served as Divisional Vice President of Global Marketing and Head of Customer and New Market Insights. Earlier in his career, Mr. Little served as Vice President of Global Cardiovascular Therapies for St. Jude Medical, where he oversaw marketing and strategy for the structural heart and vascular portfolios; Vice President, Global Marketing at Bard Peripheral Vascular; and in a variety of cardiovascular sales and marketing roles at Boston Scientific. Mr. Little holds a B.S. in Business Administration and Marketing from the University of Colorado at Boulder.
Dr. Fischer has served as Sr. Vice President (VP), Medical Affairs and Innovation for Orchestra BioMed since January 2023. Dr. Fischer brings with him extensive knowledge as a former practicing electrophysiologist along with vast industry experience in management and clinical research.
Dr. Fischer has worked with both startup and large publicly traded companies, providing him with an in-depth understanding of the unique challenges associated with development and commercialization of novel medical technologies. Immediately prior to joining Orchestra BioMed, Dr. Fischer was CEO of ElectroPhysiology Frontiers, S.p.A. in Milan. From 2012 – 2021, he worked for Abbott and St. Jude Medical where he held various leadership roles including VP, Global Education and Medical Director, Divisional VP Medical Affairs and Medical Director, and Chief Medical Officer, Cardiac Rhythm Management. Prior to joining industry, Dr. Fischer was Director, Pacemaker and Defibrillator Therapy, for Mount Sinai Medical Center in NYC from 2006 to 2017. He has lectured at national and international scientific congresses, trained and proctored physicians around the world, and authored numerous original publications, invited reviews, and book chapters. Dr. Fischer holds a Bachelor of Arts degree from the University of Maryland and a Doctor of Medicine degree from Tel Aviv University.
Mr. Faldu has served as Vice President, Pharmaceutical Development for Orchestra BioMed since January 2023. Prior to his promotion to this role, Mr. Faldu was Sr. Director, Formulation Development.
Mr. Fadlu has more than 12 years of leadership experience in program management, product development and launch supervision of complex topical and sterile drug products. Immediately prior to joining Orchestra BioMed, Mr. Faldu worked at Solaris Pharma Corporation as Sr. Director, Program Management and Manufacturing. His prior experience also includes roles of increasing responsibilities at InnoPharma and Pfizer. To date, Mr. Faldu has managed a portfolio of 25+ products and complex clinical studies and has developed and scaled up 30+ drug products including 14 that reached commercialization. Mr. Faldu received a Bachelor of Pharmacy degree from Rajiv Gandhi University of the Health Science (Bangalore, India) and a Master of Science degree in pharmaceutical manufacturing engineering from Stevens Institute of Technology.
Ms. Eileen Bailey has served as the Vice President of Quality for Orchestra BioMed since May 2018. Previously, she served in various quality, regulatory and clinical roles for Orchestra BioMed’s Interventional Therapies development group and its predecessor, Caliber Therapeutics, beginning in March 2012.
Throughout her career, she has had the opportunity to be involved in all aspects of medical device and combination device product development through commercialization. Because of her diverse technical background and expertise in regulatory quality system documentation, industry standards and cGMP she was invited to serve on the management review board for medical products at W.L. Gore and Associates, Inc. As a project manager, she has guided cross-functional teams through new product/process development and commercialization of medical devices in the fields of general surgery, bariatrics, and coronary catheters. As a functional manager, she has provided training and leadership in the areas of quality engineering and analytical/lab services. Ms. Bailey holds a MS in Polymer Engineering from University of Massachusetts and a BS in Materials Engineering from Drexel University and is a certified auditor and quality engineer with strong statistical background.
Dr. Baumbach, Ph.D. has served as Vice President, Scientific Affairs for Orchestra BioMed’s Interventional Therapies development group since May 2018. He over 30 years of experience in pharmaceutical, biotechnology, and medical device R&D.
He co-founded Caliber Therapeutics and served as its Chief Scientific Officer since 2008. Prior to Caliber, he was the Vice President of Research & Development at X-Cell Medical, a Drug-Eluting Stent startup that was based on a novel therapeutic agent, beta estradiol, that offered an enhanced safety profile. Dr. Baumbach oversaw development through a randomized first-in-human clinical trial. Prior to X-Cell, he was Director of Biology at Morphochem, a trans-Atlantic chemical genomics biotech company with pre-clinical product candidates in oncology, diabetes and anti-thrombosis from June 2000 until August 2002. From 1984 to 2000, Dr. Baumbach held a number of positions with the Molecular/Genetic Screen Design group at Wyeth Pharmaceuticals (AHP), including as Principal Research Scientist. Dr. Baumbach has over 13 years of experience in pharmaceutical research, mainly in the areas of peptide hormone receptors, GPCRs, and neurotransmitter transporters. Dr. Baumbach has published over 30 peer-reviewed articles, is an inventor on 8 issued patents, and served on the editorial board of Endocrinology, and as an adjunct professor at Rutgers. He received his Bachelor, Masters and Ph.D. degrees in Biochemistry and Molecular Biology from Princeton University.
Prior to joining Orchestra BioMed in August 2018, Mr. Belsky was Chief Technology Officer for NewPace Ltd., a cardiac rhythm management company based in Israel that is developing an implantable subcutaneous string defibrillator device for patients with atrial fibrillation.
Prior to NewPace, Mr. Belsky was a serial entrepreneur helping to found, and serving as CEO for, a number of start-up medical innovation companies in Israel, including: Novogate Medical Ltd. (2010-2013), a company focused on development of a novel means of enabling thoraco-apical access for beating heart procedures; Juvenis (2009-2010), a biomaterials company focused on aesthetic and reconstructive surgical procedures; SphinxTech Ltd. (2008-2009), a research and development company focused on medical devices for the treatment of fecal incontinence; and E-Pill Pharma Ltd. (COO, 2003-2007; CEO, 2007-2008), a company developing an oral drug delivery platform to administer peptides and large molecules (such as insulin and growth hormones), small molecules, as well as low bioavailability drugs. Previously, Mr. Belsky was Director, Business Development from 2000 to 2003 for Wavion Ltd., an Israeli company that manufactures and develops carrier-grade WiFi solutions. From 1998 to 2000, Mr. Belsky was Product Manager for Impulse Dynamics Ltd., a medical device company developing an implantable cardiac stimulator for the treatment of heart failure that was co-founded by Yuval Mika, Ph.D., our General Manager and CTO, Cardiac Neuromodulation. Mr. Belsky received his MBA from Heriot-Watt University, his MSc in electrical engineering from Tel Aviv University, and his BSc in physics and mathematics from Hebrew University in Jerusalem.
Mr. Laughner has served as Vice President, Regulatory Affairs for Orchestra BioMed since April 2020. Prior, Mr. Laughner served as Regulatory Director, Medical Device and Combination Products at AstraZeneca.
Bob Laughner has over a decade of leadership experience in combination product development and successful implementation of regulatory strategies for global medical device and biopharmaceutical companies. In his role at AstraZeneca, he managed combination product regulatory submissions and meetings with the FDA and other global regulatory agencies, while also evaluating new technologies and implementing guidelines for their development, regulatory approval and life-cycle management. Prior to AstraZeneca, Mr. Laughner served as the Associate Director, Combination Products at MedImmune, an AstraZeneca company, where he developed regulatory strategies and submissions for combination products in their biologic portfolio and developed strategies to evaluate the safety and effectiveness of drug delivery systems, including novel and wearable technologies. Prior to MedImmune, Mr. Laughner was a regulatory scientist at Cook Medical, where he provided regulatory and scientific support in the development, testing, and regulatory approval for combination products and medical devices, including many novel technologies. Mr. Laughner has been involved in the development of international standards for drug delivery devices as an expert for various ISO committees, and is currently Co-Chair of the Association for the Advancement of Medical Instrumentation’s committee on Devices for Administration of Medicinal Products and Catheters. Mr. Laughner holds an M.S. in pharmacology and a B.A. in biology from Indiana University-Bloomington and holds certification in regulatory affairs from the Regulatory Affairs Professional Society.
Mr. Lorenzo has served as Vice President, Product Development, Interventional Therapies since October 2019. Prior to joining Orchestra BioMed, Mr. Lorenzo held R&D leadership positions within different divisions of Johnson & Johnson including CERENOVUS, DePuy Synthes, Cordis Neurovascular (CNV), and Cordis Cardiology.
Mr. Lorenzo has more than three decades of experience in the medical device industry leading design, development, validation and market launches of over 47 commercial products for the treatment of various conditions within neurovascular, cardiovascular, and peripheral vascular systems. His experience includes development of neurovascular intrasaccular devices, embolic coils, flow diverters, microcatheters, balloon expandable and self-expanding stents, distal protection, PTCA catheters, guiding catheters, guidewires, and pacemakers. Mr. Lorenzo has 84 granted U.S. patents and well over 30 pending applications. Mr. Lorenzo earned a B.S. in mechanical engineering from Florida International University.
Dr. Evans has served as Medical Director, Bioelectronic Therapies for Orchestra BioMed since May 2018. Previously, he served Chief Medical Officer of BackBeat Medical under a consulting agreement since 2011.
Dr. Steven Evans, M.D. obtained his medical degree from New York University School of Medicine and completed his fellowship in Cardiovascular Disease and Electrophysiology at Cedars-Sinai Hospital. He was Director of the Electrophysiology Service at Long Island Jewish Hospital from 1990-1999 and subsequently served as Chief of Electrophysiology at Beth Israel Medical Center in New York. At both institutions, he initiated and ran successful EP Fellowship programs, participated in clinical trials, and published extensively in the field of arrhythmia detection and prediction. He is a strategic advisor in the life sciences area with 25 years of experience working in the medical device sector for companies including Biosense, Johnson & Johnson, Medtronic, Guidant, Impulse Dynamics, St. Jude, and Atricure, and is a consultant for venture funds focused on new medical technologies. His expertise spans scientific assessment, clinical research, market analysis, and regulatory and reimbursement evaluation. He is the co-inventor and co-founder of ImaCor Medical Technologies. He served as CEO of ImaCor, he secured financing and led the company through R&D, clinical trials, product manufacturing, and FDA clearance. He has also served as CEO of two oncology therapeutic companies, Althera, Inc., and Oncolyze, Inc., for which he currently serves as acting CEO and Chairman.
Mr. Sieckhaus joined Orchestra BioMed as its Vice President of Global Field Clinical Engineering in July 2024. He is an accomplished leader in the cardiovascular industry with extensive experience in field clinical engineering, commercial leadership, and medical device innovation.
Prior to joining Orchestra BioMed, Mr. Sieckhaus served as Chief Operating Officer at BioSig Technologies, where he oversaw regulatory compliance, quality assurance, contract manufacturing, and R&D initiatives for a Class II medical device technology used in electrophysiology labs. Before BioSig, he spent over two decades at St. Jude Medical, holding senior leadership roles across multiple cardiovascular product portfolios. His positions included Regional Director, Area Vice President, Divisional Vice President, and Senior Vice President of U.S. National Sales for the CRM and Electrophysiology portfolios. Following Abbott’s acquisition of St. Jude Medical, Mr. Sieckhaus was Vice President of Field Clinical Affairs, where he built and led a best-in-class team supporting more than 90 clinical trials across four business units. Earlier in his career, Mr. Sieckhaus held various positions at Intermedics, Inc., including Field Clinical Engineer. He earned a Bachelor of Science degree in Biomedical Engineering from The Johns Hopkins University.
Mr. Pomeranz joined Orchestra BioMed as Executive Vice President and General Manager of Interventional Therapies in November 2024, bringing more than 35 years of experience in the medical device industry. He has a proven track record of success, having commercialized over 50 products that have generated billions of dollars in revenue.
Prior to joining Orchestra BioMed, Mr. Pomeranz served as CEO of Motus GI, a company focused on advancing innovation in GI endoscopy. From 2007 to 2014, he was the founding CEO of Svelte Medical Systems, where he led the development of a unique drug-eluting stent platform. Before that, he spent nearly a decade at Cordis, a Johnson & Johnson company, as Vice President, where he was responsible for developing new technologies, exploring market opportunities, and leading major restructuring efforts to create cross-functional global commercialization teams. Earlier in his career, he held senior leadership roles at Cardiac Pathways Corporation and Cardiovascular Imaging Systems, both of which were later acquired by Boston Scientific Corporation.Mr. Pomeranz holds a Master of Science in Biomedical Engineering from the University of Miami and a Bachelor of Science in Mechanical Engineering from Union College. He has more than 50 issued U.S. patents across a range of medical technologies, including angioplasty catheter design, intravascular ultrasound devices, high-density cardiac mapping, embolic devices, and GI innovations. He has also authored multiple publications in electrophysiology, neurovascular treatments, and percutaneous vascular interventions.
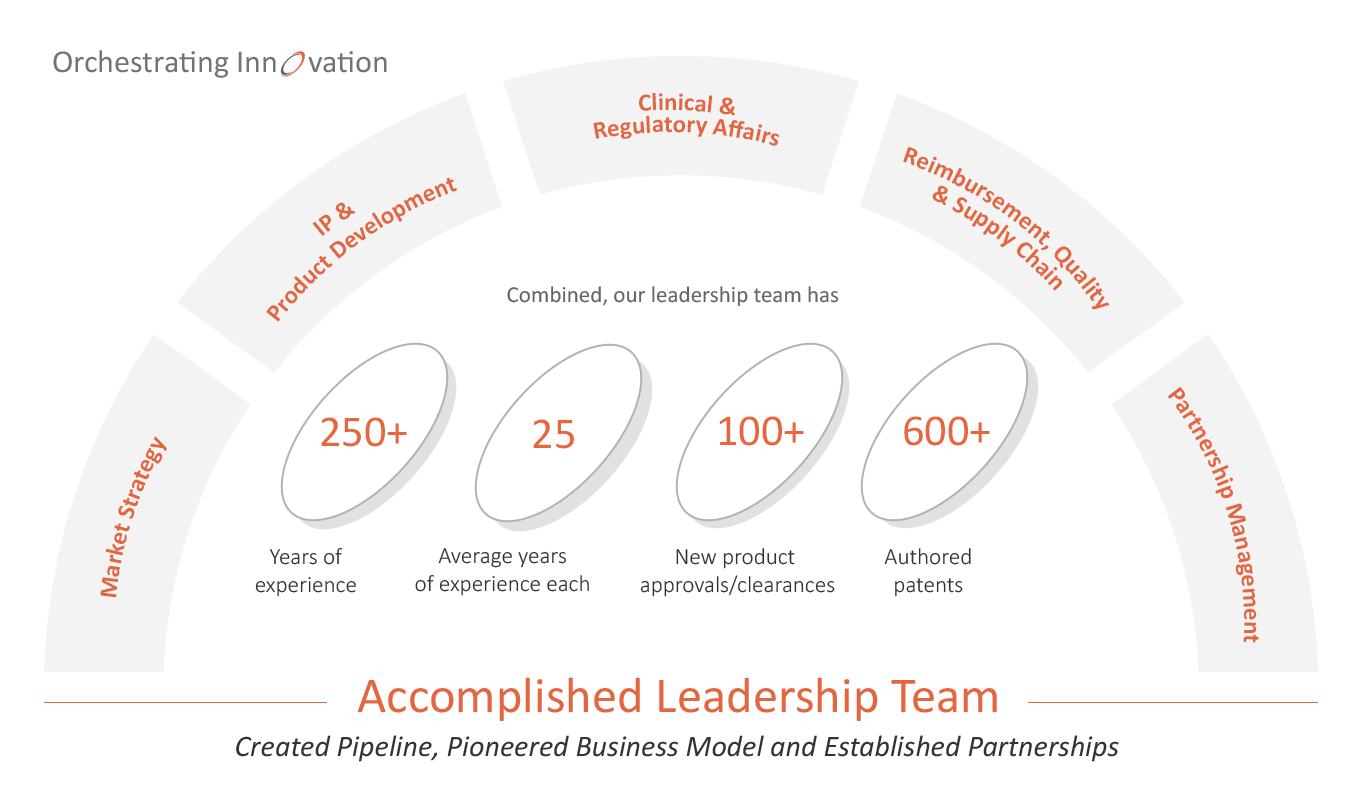
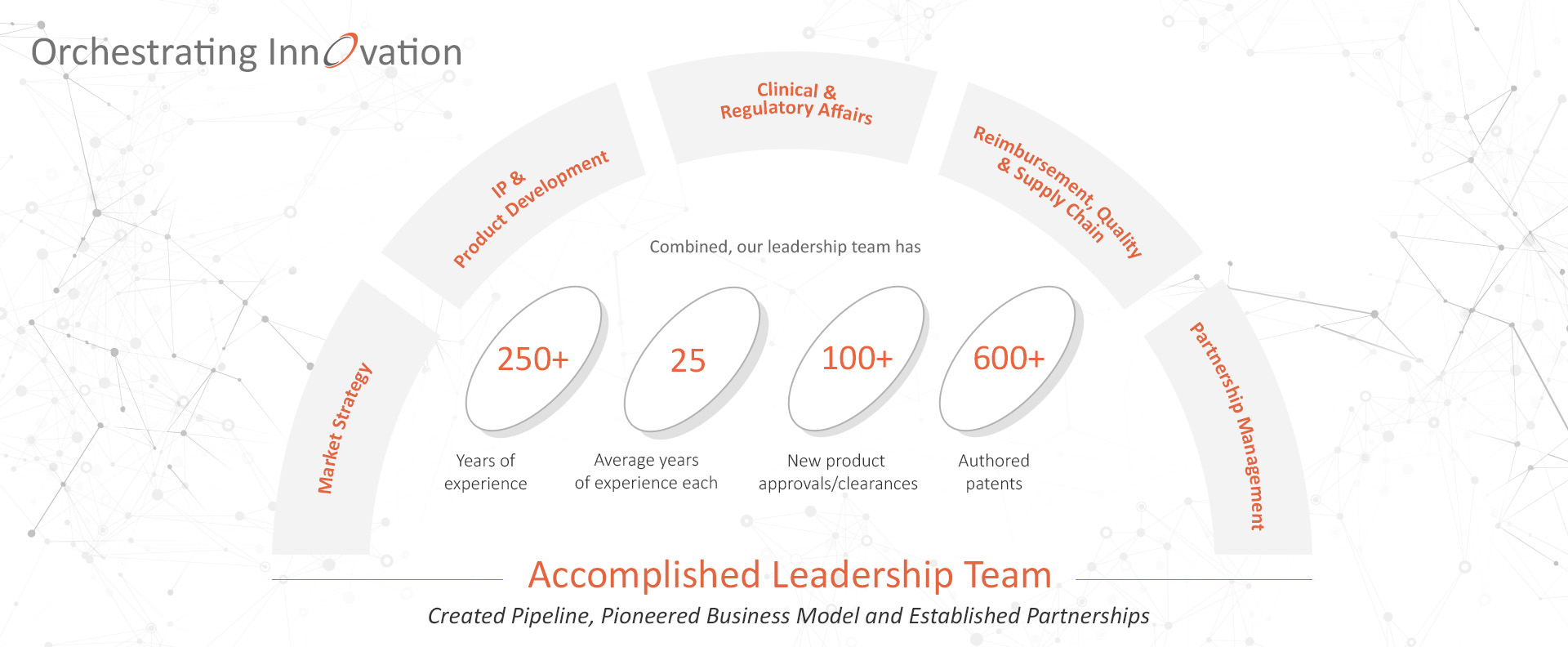
We are guided by a highly accomplished board of directors. They have extensive knowledge and experience in the healthcare industry, including medical devices, biotechnology and clinical medicine, as well as business operations, strategy, finance and capital markets.
Mr. Hochman has served as Chairman and Chief Executive Officer of Orchestra BioMed since May 2018. He previously served as Managing Partner of Orchestra Medical Ventures, an investment firm focused on creating high-impact medical device therapies.
Mr. Hochman has also served as President of Accelerated Technologies, Inc., a medical device accelerator company now owned by Orchestra BioMed. Mr. Hochman has over 25 years of healthcare entrepreneurial, venture capital and investment banking experience. From December 2016 to September 2023, Mr. Hochman served as a member of the board of directors of Motus GI (Nasdaq: MOTS), a Nasdaq listed medical technology company in which Orchestra has a strategic investment and as its Chairman of the Board from December 2016 to April 2023. Mr. Hochman was a co-founder and until September 2020 served as a board member of Corbus Pharmaceuticals Holdings, Inc. (Nasdaq: CRBP), a clinical-stage company focused on the development and commercialization of novel medicines designed to target the endocannabinoid system. Prior to joining Orchestra Medical Ventures, Mr. Hochman was Chief Executive Officer of Spencer Trask Edison Partners, LLC, an investment partnership focused on early-stage healthcare companies. He was also Managing Director of Spencer Trask Ventures, Inc. during which time he led financing transactions for over twenty early-stage companies raising over $420 million. From 1999 to 2006, Mr. Hochman was a board advisor of Health Dialog Services Corporation, a leader in collaborative healthcare management that was acquired in 2008 by the British United Provident Association for $750 million. From 2005 to 2007, he was a co-founder and board member of PROLOR Biotech, Inc., a biopharmaceutical company developing longer lasting versions of approved therapeutic proteins, which was purchased by Opko Health (NYSE: OPK) in 2013 for over $600 million. Mr. Hochman earned a B.A. with honors from the University of Michigan.
Mr. Sherman has served as President, Chief Operating Officer and a member of the Board of Orchestra BioMed since May 2018. From 2009 until December 2019, Mr. Sherman served as Managing Partner of Orchestra Medical Ventures, an investment firm focused on creating high-impact medical device therapies. He co-founded Orchestra BioMed and its wholly owned subsidiaries Caliber, BackBeat and Freehold.
Mr. Sherman also served as Chief Technical Officer of Accelerated Technologies, Inc. (ATI), a medical device accelerator company now owned by Orchestra BioMed. Mr. Sherman has over 25 years of management and entrepreneurial experience in the medical technology industry spanning interventional cardiology, cardiac electrophysiology, sudden cardiac death, stroke, surgery, GI, and neurovascular therapies. Mr. Sherman served as Chief Executive Officer and a Director of Caliber since 2009. He was a co-founder and served on the Board of BackBeat since 2010. He was a co-founder and served as Chief Executive Officer and a director of FreeHold since 2011. From 2009 until 2016, he served on the board of directors of Vivasure. He served as a director of Motus GI (Nasdaq: MOTS), a Nasdaq-listed medical technology company that Orchestra also has a strategic holding in, from December 2016 to September 2023. From February 2002 until March 2008, Mr. Sherman held various positions in executive management for Cordis Neurovascular (CNV), a Johnson & Johnson company, including Executive Director R&D and Director of Strategic Marketing for stroke products. He had worldwide responsibility for all neurovascular R&D and global strategic marketing responsibilities for the stroke franchise, including budgets, portfolio of products and strategic planning. Mr. Sherman made significant contributions to the design and commercialization of a series of key products including the Enterprise Vascular Reconstruction Device and the Orbit Embolic Coil. From January 1997 until February 2002, Mr. Sherman played an integral role in the formation and development of Revivant Corp (acquired by Zoll) while working with Thomas J. Fogarty, M.D. at Fogarty Engineering. He was Revivant’s first employee and managed the design, development, and testing of the AutoPulse device from concept through market introduction. In addition to the R&D related activities, Mr. Sherman supported the clinical research, marketing, and intellectual property efforts. The AutoPulse is a lightweight, portable tool replacing the need for manual chest compressions during cardio-pulmonary resuscitation (CPR) for patients suffering from sudden cardiac arrest. From January 1995 until January 1997, Mr. Sherman held positions in research and development for Cardiac Pathways Corp., prior to its acquisition by Boston Scientific. Prior to Cardiac Pathways, he worked at Baxter Healthcare. In each of these companies, he participated in the creation, development and launch of products. Mr. Sherman has authored more than 85 U.S. patents with an additional 90+ published applications. He earned a BS degree in Bioengineering from the University of California, San Diego.
Dr. Fain became a member of Orchestra BioMed’s Board of Directors in November 2018. He previously served as a Strategic Advisor and a consultant to BackBeat Medical, LLC. since October 2017.
Since July 2018, he has served as the President and CEO of Procyrion, Inc., a development stage medical device company developing catheter-based circulatory support technologies for congestive heart failure patients. Previously, he was the Senior Vice President and Group President, Cardiovascular and Neuromodulation at Abbott following its acquisition of St. Jude Medical. Dr. Fain became Group President of St. Jude Medical in 2014 where he was responsible for global sales, marketing and clinical affairs across the entire St. Jude Medical portfolio worldwide. Dr. Fain joined St. Jude Medical in 1997 as Vice President, Systems Development as part of the company’s acquisition of Ventritex, Inc., where he had worked since 1987. He went on to become Senior Vice President, Clinical/Regulatory Affairs for the St. Jude Medical Cardiac Rhythm Management Division (CRMD) in 1998. He was later promoted to Executive Vice President, Development and Clinical/Regulatory Affairs for CRMD in 2005 and then President of CRMD in 2007. Prior to his role as Group President, Dr. Fain was President of the company’s Implantable Electronic Systems Division beginning in 2012 when its CRM and Neuromodulation Divisions merged. Dr. Fain received his M.D. from Stanford University School of Medicine and holds a Sc.B. degree in applied math/biology from Brown University.
Jason Aryeh has served on Orchestra BioMed's Board of Directors since November 2018. He serves as Chairman of Orchestra’s Nominating & Governance and on its Compensation Committee.
Mr. Aryeh has more than 25 years of equity investment experience focused on the life sciences industry. He is the Founder and Managing General Partner of JALAA Equities, LP, a private investment fund focused on the biotechnology and medical device sectors. He has served in such capacity since 1997. In addition to the Orchestra's Board, Mr. Aryeh currently serves on the Board of Directors of Ligand Pharmaceuticals (NASDQ: LGND), LifeCore Biomedical (NASDQ: LFCR), and Anebulo Pharmaceuticals (NASDQ: ANEB). He serves as Chairman of Ligand’s Nominating & Governance and on its Compensation and Strategic Committees, on Lifecore’s Nominating & Governance Committee, and as Chairman of Anebulo’s Nominating & Governance and on its Audit and Strategic Committees. Since 2006, Mr. Aryeh has served as Chairman of the Board, on the Board of Directors or as a consultant to over fifteen public and private life sciences companies and charitable foundations, including the Cystic Fibrosis Foundation’s Therapeutics Board for seven years. Mr. Aryeh earned a B.A. in economics, with honors, from Colgate University, and is a member of the Omnicron Delta Epsilon Honor Society in economics.
Ms. Connealy became a member of Orchestra BioMed’s Board of Directors in February 2020. She serves as the Audit Committee Chair.
Ms. Connealy is the chief financial officer of Pyxis Oncology, a diversified oncology company focused on developing an arsenal of next-generation therapeutics to target difficult-to-treat cancers and improve quality of life for patients. At Pyxis Oncology, Ms. Connealy leads finance, investor relations, legal and human resources. Ms. Connealy has over 25 years of finance and business operations experience and prior to joining Pyxis Oncology Pam was the CFO, Chief HR Officer of Immunovant with additional responsibilities for legal, IT and facilities. Pam also served as the Chief Financial Officer and Chief Operating Officer of Kiva, a San Francisco based nonprofit organization. From April 2014 to June 2018, Pam served as Global Head of Talent at the Bill & Melinda Gates Foundation, focusing on talent management, compensation, benefits, and global mobility. From March 2012 to November 2013, she served as Vice President of Business Operations at Salesforce, and from March 2002 to April 2010, Pam served as a Vice President and Corporate Officer at Genentech, with roles including Chief Financial Officer of Research & Development and Global Head of Procurement. Pam earned a B.S. in Chemistry from Gannon University and an M.B.A. in Finance from the University of St. Thomas in Houston, Texas.
David Pacitti, MBA, became a member of Orchestra BioMed’s Board of Directors in March 2024. He serves as a member of the Compensation and Audit Committees. He is currently President and Chief Executive Officer of Avanos Medical, Inc.
Prior to joining Avanos, Mr. Pacitti was President of Siemens Medical Solutions USA, Inc. and Head of the Americas, Siemens Healthineers. In this role, he oversaw the company’s business in North and Latin America, leading marketing, sales, service, and support functions across its full portfolio, including medical imaging, laboratory diagnostics, therapy solutions, and services. Mr. Pacitti was also Division Vice President of U.S. Commercial Operations, Sales, and Marketing at Abbott Vascular for two years, overseeing the company’s business in North America. As a member of the Senior Executive Staff, he worked with the CEO, CFO, and Research & Development team on business development initiatives and played pivotal roles in key launches, including Abbott Vascular’s first drug-eluting stent franchise and structural heart franchise. Prior to this position, Mr. Pacitti was Vice President of Abbott Vascular’s Commercial Operations from 2009 to 2013, and Vice President of Global Marketing from 2006 to 2009. He joined Abbott Vascular with its acquisition of Guidant Corp, where he held several roles from 1995 to 2006. Early in Pacitti’s career, he was a sales representative in Siemens’ Molecular Imaging business. Mr. Pacitti is Board Chair for the Advanced Medical Technology Association (AdvaMed) and the Siemens Foundation. He is also a member of the CEO Council for Growth at the Chamber of Commerce for Greater Philadelphia, the Children’s Hospital of Philadelphia Corporate Council, the Medical University of South Carolina (MUSC) President’s Advisory Group, and the NextGen Advisory Board for the University of Missouri.
Mr. Mack became a member of Orchestra BioMed’s Board of Directors in July 2024.
Mr. Mack most recently served as President of Cardiac Surgery at Medtronic plc., where he successfully led the division to market leadership. During his 26-year tenure at Medtronic, he also served as Vice President & General Manager, Extracorporeal Therapies and Vice President, Business Development, Strategy & Portfolio Management where he led the business development operations for the coronary and structural heart business, a $3.2B global enterprise with over 12,000 employees. Mr. Mack has extensive board experience, including currently serving as a board member for the Minneapolis Heart Institute Foundation. Previously, Mr. Mack served on the boards of MC3 Cardiopulmonary and the Twin Cities American Heart Association.
Mr. Cleary became a member of Orchestra BioMed’s Board of Directors in February 2025.
Mr. Cleary most recently served as Senior Vice President of Corporate Development at Medtronic, where he facilitated over 35 acquisitions, multiple structured and venture investments, and orchestrated the $6 billion sale of Covidien Group S.à.r.l's medical supply assets to Cardinal Health Inc. (NYSE: CAH). His strategic acumen and negotiation skills played a key role in driving Medtronic’s growth and market expansion. Prior to Medtronic, Mr. Cleary was CEO of Alesia Capital Services, a management consulting firm advising Fortune 500 companies, including Medtronic, Goldman Sachs, Ally, Macquarie Capital, and Terex. He also led M&A teams within several businesses at GE Capital, closing acquisitions totaling more than $60 billion across 200+ transactions in the US, Canada, Europe, Asia, and Latin America. Mr. Cleary currently serves on the boards of Enterra Medical, Inc. and Pristine Surgical LLC. He earned his B.A. in Biology from Colorado College.
Our product development efforts are supported by world-renowned medical advisors, who are physicians and scientists recognized for their knowledge of specific disease states and treatment options, as well as their ability to assess new technologies for clinical feasibility and likelihood of adoption.
Dr. Angelo Auricchio serves as the Director of the Heart Failure Program and Clinical Electrophysiology Unit at the Fondazione Cardiocentro Ticino in Lugano, Switzerland.
His focus is on cardiac catheterization and cardiac electrophysiology. His main areas of research are in clinical electrophysiology - mapping techniques in dilated cardiomyopathies, non-pharmacological therapy of arrhythmias - and non-pharmacological therapies for heart failure. Moreover, he serves as a Co-Director at the Center for Computational Medicine in Cardiology at Università della Svizzera Italiana in Lugano, Switzerland. He has been Professor of Cardiology at University Hospital in Magdeburg, Germany since 2008. He also serves as Scientific Director at the Fondazione Ticino Cuore. Dr. Auricchio is also a part-time professor in Cardiology at the University of L’Aquila (Italy). He was the President of the European Heart Rhythm Association (EHRA). Dr. Auricchio is the author of more than 250 peer reviewed articles, 6 books and more than 20 book chapters. Dr. Auricchio received his medical degree from medical school in Naples (Italy) and a Ph. D. in Cardiovascular Physiology from the University of Rome “Tor Vergata” (Italy). He completed his fellowship in Cardiology at Federico II - University of Naples, Italy.
Dr. Barry Katzen is Voluntary Professor of Radiology at the University of Miami School of Medicine as well as Clinical Professor of Radiology at the University of South Florida College of Medicine, and most recently became Associate Dean for Clinical Affairs at the Florida International University Herbert Wertheim College of Medicine.
Dr. Katzen is considered a pioneer of angioplasty thrombolysis and has a particular interest in treatment of aortic (AAA, TAA) and peripheral aneurysms, peripheral arterial disease (PAD) and critical limb ischemia, carotid artery disease, renal vascular hypertension, and vascular malformations. He has participated in the innovation of new therapies for a number of vascular diseases. He is a program director for the International Symposium on Endovascular Therapy (ISET) and Symposium on Clinical Interventional Oncology (CIO). Dr. Katzen received his medical degree from the University of Miami School of Medicine. He completed his radiology residency at the New York Hospital-Cornell Medical Center, with a fellowship in cardiovascular radiology at St. Vincent’s Hospital Medical Center in New York, as well as the University of Rome, Italy.
Dr. Burkhoff is currently Director of Heart Failure, Hemodynamics and MCS Research at the Cardiovascular Research Foundation, serves as a consultant to several heart-failure device companies and is also Adjunct Associate Professor of Medicine at Columbia University Medical School.
He is also the Founder of PVLoops, a cardiovascular educational software company. He is also creator of Harvi, an interactive simulation-based mobile application available for the iPad for teaching and researching the aspects of ventricular mechanics and hemodynamics. After earning a B.S. degree from Cornell University in Applied and Engineering Physics, Dr. Burkhoff obtained Ph.D. and medical degrees from The Johns Hopkins School of Medicine and completed a fellowship in Cardiology at The Johns Hopkins Hospital. In 1992, Dr. Burkhoff became an Associate Professor at the Columbia University School of Medicine and worked at Columbia University Medical Center where he ran the Cardiovascular Research Laboratory through 2003. Dr. Burkhoff then became the Director of the Jack Skirball Center for Cardiovascular Research of the Cardiovascular Research Foundation through 2005. In conjunction with and following this role, Dr. Burkhoff served as the Medical Director of several startup companies, including Impulse Dynamics, Inc. (2003-2014), a company focused on an implantable cardiac stimulator for the treatment of heart failure, Cheetah Medical, Inc. (2006-2012), an advanced hemodynamic monitoring technology company, as well as CircuLite (2009-2013), which he co-founded and which was acquired by HeartWare in 2013. He served as Vice President, Medical Science for Heartware until it was purchased in 2016 by Medtronic for $1.9 billion. Dr. Burkhoff’s expertise includes cardiovascular modeling and research in basic and clinical aspects of ventricular mechanics, cardiovascular monitoring, heart failure, device and pharmacologic treatments for heart failure, including left ventricular assist devices.
Dr David E. Kandzari is the Director, Interventional Cardiology and Chief Scientific Officer the Piedmont Heart Institute in Atlanta, Georgia. Dr Kandzari specializes in cardiovascular disease, peripheral arterial disease and interventional cardiology.
A graduate of Duke University School of Medicine, he completed his internship and residency at The John Hopkins University School of Medicine in Baltimore, Maryland. Following his residency, he completed his general and interventional cardiology fellowship at Duke University where he joined the faculty as the John B. Simpson Assistant Professor of Interventional Cardiology and Genomic Sciences. Dr Kandzari is the former Director of Interventional Cardiology Research at the Scripps Clinic in La Jolla, California. Before joining the Scripps faculty, he also served as Chief Medical Officer for the Cordis Corporation, a Johnson & Johnson Company. He has also served as a Medical Officer for the Center for Devices and Radiological Health for the United States Food and Drug. Board certified by the American Board of Internal Medicine and its subspecialty Board of Cardiovascular Disease and Board of Interventional Cardiology, Dr Kandzari has held national and international leadership roles in clinical trials in cardiovascular disease and has participated in national and international program committees in cardiology. He has authored and coauthored more than 200 studies, book chapters and scientific reviews.
Dr. Dean J. Kereiakes is Medical Director of The Christ Hospital Heart and Vascular Center, Medical Director of the Carl and Edyth Lindner Center for Research and Education at The Christ Hospital, and Professor of Clinical Medicine at Ohio State University.
Dr. Kereiakes has been an original investigator for most of the interventional technologies introduced in the last two decades and has performed more than 30,000 catheterization procedures. In addition to lecturing nationally and internationally, Dr. Kereiakes is very active as a clinical and scientific investigator and has participated in over 1,300 clinical research protocols. He has published over 800 journal articles, abstracts and book chapters. He has served on the numerous editorial boards of prestigious, peer-reviewed journals, including Circulation, The Journal of the American College of Cardiology (JACC). Dr. Kereiakes received his medical degree and was valedictorian of his graduating class at the University of Cincinnati in Ohio. His postgraduate training included internship and residency at the University of California, San Francisco, a senior residency at Massachusetts General Hospital in Boston, and a chief residency at the University of California, San Francisco. He then completed fellowships in adult cardiology at the University of California, San Francisco and in coronary angioplasty at the San Francisco Heart Institute and the Sequoia Hospital.
Dr. Gregg W. Stone is a Professor of Medicine at Columbia University. He is also the Director of Cardiovascular Research and Education at New York Presbyterian Hospital/ Columbia University Medical Center and is a Co-Director of Medical Research and Education at The Cardiovascular Research Foundation, New York, NY.
Dr. Stone is an expert in cardiovascular research. His expertise includes; design and conductance of clinical trials specifically trials related to treatment of hypertension. Dr. Stone participates in the scientific advisory board of numerous cardiovascular trials including leading device trials for the treatment of hypertension. Dr. Stone is the founder of TCTMD.com, Associate Editor, JACC Cardiovascular Imaging and on the editorial board of many cardiovascular journals. Dr. Stone served in the medical and scientific advisory board of more than 100 clinical studies. Dr. Stone is the author of hundreds of Journal articles and the author of 34 books and book chapters. Dr. Stone received his medical degree from The Johns Hopkins School of Medicine Baltimore, MD. He completed his internship and residency, at the New York Hospital-Cornell Medical Center, New York City, NY; fellowship in Cardiology at Cedars-Sinai Medical Center, Los Angeles, CA and fellowship in Advanced Coronary Angioplasty at Mid America Heart Institute St. Luke's Hospital Kansas City, MO.
Dr. Stephen Scott is a board-certified surgeon with the Surgical Weight Loss Institute of Kansas City. He is affiliated with Lee’s Summit Medical Center.
Dr. Scott offers his patients a breadth of experience and expertise in bariatric surgery, having worked in the field for more than 20 years. During that time, he has seen complications decrease ten-fold, and has played an important role in that improvement through his involvement in training, education, new techniques and technologies. Most recently, Dr. Scott served as an associate professor in general surgery for the University of Missouri at Columbia, and co-director of Missouri Bariatric Services. He has also served on the clinical faculty of schools including St. Louis University and the University of Health Sciences College of Osteopathic Medicine, and on the faculty of the Advanced Laparoscopic Training Center in Atlanta. Dr. Scott is an active researcher, presenter and author, and possesses a level of knowledge in his field that ensures patients receive the latest, most advanced bariatric care. He recently authored 3 text book chapters on the topic. In addition to his medical training, Dr. Scott is the co-founder and co-director of Towertech Research Group, a medical technique/procedure research entity. He has served on the boards of numerous entities including the American Hernia Society, the Ethicon Endo-Surgery Institute and on advisory boards for General Surgery Innovations and U.S. Surgical Corp. Dr. Scott has been the director of the Robotic Program at American Society of Metabolic and Bariatric Surgeons, moderator of the robotic session at Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) forums, spoken extensively on revisional bariatric surgery at SAGES, the American Society of Metabolic and Bariatric Surgery (ASMBS), the American College of Surgeons (ACS) and surgical disruptive technologies conferences. He holds memberships in the International Federation for the Surgery of Obesity and Metabolic Disorders, the International College of Surgeons, ASMBS, the American Hernia Society, the American Society of General Surgeons, the ACS, and SAGES.
Dr. Jagmeet P. Singh is the Associate Chief of the Cardiology Division at the Massachusetts General Hospital and Professor of Medicine at Harvard Medical School. He is the Founding Director of the Resynchronization and Advanced Cardiac Therapeutics Program.
Dr. Singh is the Deputy editor of the Journal of American College of Cardiology: Clinical EP, the editor in chief of Current Treatment Options in Cardiovascular Medicine, was the international associate editor of European Heart Journal and is in the editorial board of: EP Lab Digest and Journal of Innovation in Cardiac Rhythm Management. Dr. Singh serves on the steering committee and scientific advisory board of numerous national and international clinical trials mainly Dr. Singh is the author of more than 250 Journal articles and the author of 4 books and book chapters. Dr. Singh received his medical degree from B.J. Medical College, Pune University, India. He completed his Ph.D. studies at Oxford University, his residency and fellowship at Massachusetts General Hospital.
Dr. John M. Flack is a professor and Chair of the Department of Internal Medicine at Southern Illinois University School of Medicine.
Prior to joining SIU, Dr. Flack held numerous positions at Wayne State University School of Medicine in Detroit, most recently as professor of medicine and physiology. He was chair of Wayne State’s internal medicine department for 8 years. While at Wayne State, Flack served as the founding director of the Division of Translational Research and Clinical Epidemiology (TRaCE) and was principal investigator of the National Institute of Environmental Health Sciences (NIEHS)-funded Center for Urban Health. Dr. Flack serves as the Vice President of the ASH Hypertension Specialist Board. Dr. Flack is the prior President of International Society on Hypertension in Blacks (ISHIB) and is also past member, FDA Cardio-renal Advisory Panel. Dr. Flack is the author of more than 180 publications and 18 books and book chapters. Dr. Flack is an Associate Editor of American Journal of Hypertension and CardioRenal Medicine. Dr. Flack received his medical degree from the University of Oklahoma Health Sciences Center. He completed his internship and residency at the University of Oklahoma Health Sciences Center and NIH post-doctoral fellowship in cardiovascular epidemiology at University of Minnesota.
Dr. Juan Granada is President and Chief Executive Officer of the Cardiovascular Research Foundation and was previously the Executive Director and Chief Innovation Officer of the CRF Skirball Center for Innovation.
He is also an Assistant Professor of Medicine at Columbia University College of Physicians and Surgeons and a Co-Director of TCT. Dr. Granada is one of the world’s foremost investigators in the field of translational research involving advanced experimental methodologies, endovascular imaging and emerging cardiovascular therapies. He has helped to develop and successfully validate several endovascular imaging and cardiovascular device technologies currently in clinical use worldwide. In addition, he is an active participant in the development of multiple innovation programs around the world. Dr. Granada received his medical degree from the Institute of Health Sciences in Medellin, Colombia. He completed his cardiovascular disease and interventional cardiology fellowships at Baylor College of Medicine in Houston, Texas. He joined CRF in 2007 from the Methodist Hospital Research Institute in Houston, Texas.
Dr. Karl-Heintz Kuck serves as a Director of Cardiology of Allegemienes Krankenhaus St. Georg, Hamburg, Germany. His main research interests include: Clinical Electrophysiology, Valvular Heart Disease, Percutaneous Catheter Intervention, Catheter Based Cardiac Stem Cell Therapy, Cardiac Devices in Heart Failure and Atrial Fibrillation. Dr. Kuck serve on the advisory board of several Medical device companies.
Dr. Kuck has served as a Board Member of the European Heart Rhythm Association (EHRA) for the past 4 years and serves as EHRA President. Dr. Kuck was the president German Cardiac Society (GCS) and a board member of the European society of cardiology (ESC). Dr. Kuck is the author of more than 400 publications, many books and book chapters and scientific reviews. Dr. Kuck serves on the editorial boards of the German Journal of cardiology, the Chinese Journal of Cardiology and the Journal of Cardiovascular Electrophysiology. Dr. Kuck received his medical degree from the University of Cologne, Germany. He completed his internship in Department of Cardiology of the University Hospital Eppendorf (UHE) Hamburg, Germany and his fellowship at the department of Clinical Electrophysiology of the University Hospital Limburg in Maastricht, Netherlands.
Dr. Paul Teirstein is the Chief of Cardiology and Director of Interventional Cardiology for Scripps Clinic and Director of the Scripps Prebys Cardiovascular Institute for Scripps Health. Dr. Teirstein joined Scripps in 1987 and is the founder and director of the interventional cardiology program at Scripps Clinic.
Through this program he is leading the way in training a new generation of interventional cardiologists. With a primary focus on complex coronary interventions and new technology development, Dr. Teirstein has played an active role in the initial development and clinical investigation of coronary stent procedures, rotablator atherectomy, transluminal extraction atherectomy, coronary angioscopy and the utilization of cardiopulmonary support. Dr. Teirstein pioneered the first effective treatment for restenosis (low dose radiation therapy) and was one of the early investigators of medicated stents. Recent areas of investigation include new technology for minimally invasive transcatheter aortic valve replacement (PARTNER trial) and transcatheter treatment of hypertension (Simplicity trial). The author of numerous publications, Dr. Teirstein has lectured throughout the world on state-of-the-art interventional cardiology techniques. He is an internationally recognized leader in cardiology and has received numerous awards for both research and clinical excellence. Dr. Teirstein completed medical school at Mount Sinai School of Medicine followed by internship and residency at Brigham and Women’s Hospital. He completed his fellowships St Luke’s Mid America Heart Institute and Stanford University Hospital.
Dr. Pieter Stella was trained as a cardiologist in the OLVG-Amsterdam and did his fellowship in interventional cardiology at the University of Utrecht. Since 1997, Dr. Stella has practiced as an interventional cardiologist at the University of Utrecht, having performed more than 6,500 PCI procedures.
He has served as director of the Cat labs from 2014-2017. In 2017, Dr. Stella was appointed Research Manager for the Division of Heart & Lungs of the University Medical Center Utrecht. Dr. Stella has been involved in many trials as principal investigator and has authored more than 140 peer-reviewed publications. He has also been invited to be a guest-faculty at major conferences such as Euro-PCR, TCT, Asia-live, ICI, CIT, CRF, India live and has also conducted local proctoring in Europe, Asia/China and the US. Dr. Stella’s current interests include reduction of DAPT with new stents, new stent designs and Transcatheter Valve techniques. He is a visiting professor of Medicine at the National University of Singapore and University of Sozhou in China.
Dr. Robert S. Schwartz is Medical Director of the Minnesota Cardiovascular Research Institute and is Senior Cardiologist at the Minneapolis Heart Institute.
He was previously Director of the Center for Applied Vascular Biology and Interventions, Mayo Foundation. Dr. Schwartz has also served as Professor at the Mayo Medical School. He holds Board Certifications on the National Board of Medical Examiners in Internal Medicine, the American Board of Internal Medicine in Cardiovascular Diseases, and the Society of Cardiovascular Computed Tomography (SCCT). Dr. Schwartz has published over 500 articles and abstracts in peer reviewed journals dealing with various topics in Interventional Cardiology and Advanced Cardiac Imaging. He is the recipient of various awards including the Andreas Gruentzig Award for Basic Research in Coronary Restenosis from the Thoraxcenter/European Society of Cardiology. He holds professional memberships as Fellow of the American College of Cardiology, American Heart Association, and The Society for Cardiac Angiography and Interventions. Dr. Schwartz is also a member of the Society of Atherosclerosis Imaging. Dr. Schwartz received a Bachelor’s degree in Engineering Physics, a Master’s degree in Electrical Engineering, and his doctorate from the University of Colorado-Denver and continued his internship, residency, and Fellowship at the Mayo Graduate School of Medicine.
Dr. Roger de la Torre is a board-certified surgeon with the Surgical Weight Loss Institute of Kansas City. He is affiliated with Menorah Medical Center.
Most recently, Dr. de la Torre served as an associate professor and director of the Bariatric Program at the University of Missouri at Columbia, and as director of the school's BioDesign and Innovation Program. He has also served on the faculty of schools including University of Health Sciences College of Osteopathic Medicine and St. Louis University, and on the faculty of the Advanced Laparoscopic Training Center in Atlanta. Dr. de la Torre offers his patients a wealth of experience and expertise in bariatric surgery, having worked in the field for more than 15 years. He has been the recipient of numerous awards, most notably a U.S. News and World Report High Performing Specialty Award, along with others recognizing his surgical and overall care skills. Dr. de la Torre has been an active researcher and author, writing multiple papers, abstracts and articles detailing advances and methods in bariatric and laparoscopic surgical techniques. His level of knowledge in this area ensures patients receive the latest most advanced care in bariatric and minimally invasive procedures. In addition to his medical training, Dr. de la Torre holds over 65 patents, and was recently inducted into the National Academy of Inventors. He is the co-founder and director of Towertech Research Group, a medical technique/procedure research entity. He is listed among Who’s Who of Science and Engineering and Who’s Who of American Inventors. Dr. de la Torre's professional memberships include the American College of Surgeons, the International College of Surgeons, the Society of Laparoendoscopic Surgeons, the Society of American Gastrointestinal Endoscopic Surgeons, the American Society for Metabolic & Bariatric Surgery, and the American Society of General Surgeons. In 2021, Dr. de la Torre was named the unanimous winner of the ASMBS Foundation's Surgical Innovation Award.
Dr. Verheye has been a senior interventional cardiologist at the Antwerp Cardiovascular Center, Antwerp Belgium since 2000. He serves as a member of Scientific Advisory Board of several medical device manufacturers.
He is a member of the European Association of Percutaneous Cardiovascular Interventions and a member of the Belgian Working Group on Interventional Cardiology, and has been appointed as Visiting Professor at the University of Brussels. Dr. Verheye is the author of over 150 peer-reviewed journals (www.pubmed.com), ten book chapters, over 300 abstracts, and has led (as global principal investigator) and participated in numerous clinical (Phase I and II) and pre-clinical research studies in the field of innovative interventional cardiology (coronary and structural heart disease). He has presented at numerous major conferences such as TCT (Transcatheter Cardiovascular Therapeutics), EuroPCR (European Percutaneous Course on Revascularization), AsiaPCR, AHA (Amercian Heart Association), ACC (American College of Cardiology) and ESC (European Society of Cardiology) and has directed and transmitted several coronary and structural live cases to TCT, EuroPCR and ESC. His entrepreneurial interests led to the co-foundation of Hilbert Paradox, NV, a start-up company encompassing a unique way of capturing and correlating digital health data, applying data- and knowledge engineering, which was later on acquired by a clinical research organization. Afterwards he co-founded ainthoven, NV, a start-up company dedicated to on-line cardiac signal analytics by exploring the hyperspace of learning algorithms.
Dr. Vivek Y. Reddy is one of the nation’s premier cardiac electrophysiologists, leading a team of physician-scientists who are developing and testing advanced therapies for cardiac arrhythmias.
Dr. Reddy’s pioneering clinical research is changing the way cardiac patients are treated and cured. Under his leadership, Mount Sinai is the lead investigator on several multinational clinical trials exploring new arrhythmia procedures and technologies. Dr. Reddy is the author of more than 200 Journal articles and the author of 6 books and book chapters. Dr. Reddy received his medical degree from the Univ. of Michigan Medical School. He completed his residency in Internal Medicine at Yale-New Haven Hospital, his fellowship in Cardiovascular Discipline at University of Chicago Hospitals and his fellowship in electrophysiology at Massachusetts General Hospital.
© 2025 Orchestra BioMed Inc. Virtue®, BackBeat CNT™ FreeHold Duo®, FreeHold Trio® and Orchestra BioMed™ are trademarks of Orchestra BioMed.
All other trademarks are trademarks of their respective owners.
SM-0020 Rev 01